Metal and Non Metal
•Elements can be Classified as metals and non-metals on the basis of their properties.
•Example of some metals are:
Iron(Fe), Aluminium(Al) Silver(Ag), Copper(Cu)
•Example of some non-metals are:
Hydrogen(H), Nitrogen(N), Sulphure(S)
,Oxygen(O)
difference between metal and non metal
Physical properties of metals
1.lustre:Metals have shining surface.
2.Hardness:They are generally hard.Except sodium,lithium and Potassium which are soft and can be cut with knife.
3.state:Exist as solids.Except Mercury.
4.Malleability:Metals can be beaten into thin sheets.gold and silver are the most malleable metals.
5.Ductility:Metals can be drawn into thin wires.
6.Conductor of heat and electricity:Metals are good conductors of heat and electricity.Silver(Ag) and copper(Cu):Best conductors of heat.lead(Pb), mercury(Hg)poor conductor of heat.
7.Density:Generally have high density and high melting point.Except sodium and Potassium.
8.Sonorous:Metals produce a sound on striking a hard surface.
9.Oxides:Metallic oxides are basic in nature.
Physical Properties of non-metal
1.lustre: They do not shining surface.Except iodine.
2.Hardness: Generally soft .Except Diamond , a form of carbon which is the hardest nature substance.
3.State: Exist as solids or gaseous.Except bromine.
4.Malleability: Non-metals are non-malleable.
5.Ductility:They are non - ductile.
6.Conductor of heat and electricity:Non-metals are poor conductor of heat and electricity.Except graphite.
7.Density:Have low density and low melting point.
8.Sonorous:They are not sonorous.
9.Oxides:Non-metallic oxide are acidic in nature.
(II) Chemical Properties of metals
(a) Reaction with Air:
Metals combine with oxygen to form metal oxide.
Metal +0²→Metal oxide.
Examples:-
(1) 2Cu+O²→2CuO(copper oxide)
[Black]
(2)4Al+3O²→2Al²O³ (Aluminium oxide)
(3)2Mg+O²→2Mgo
Some important things or points keep.
•Different metals show different activities towards O².
•Na and K react to so vigorously that they catch fire if kept in open so they are kept immersed in kerosene.
•Surfaces of Mg, Al, Zn, Pb are covered with a thin layer of oxide which prevent them from further oxidation.
•Fe does not burn on heating but iron fillings burn vigorously.
•Cu does not burn but is coated with black copper oxide.
•Au and Ag does not react with oxygen.
Amphoteric oxides: Metal oxides which react with both acid as well as bases to produces salts and water are called amphoteric oxide.
Examples:Al²O³+6HCl→2AlCl³+H²0
Al²O³+2NaOH→2NaAlO²→H²O
(Sodium Aluminium)
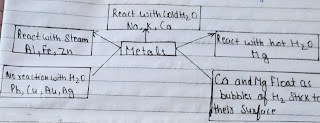
(b)Reaction of Metals with water:
Metal+water→Metal oxide+Hydrogen
Metal oxide+water→Metal hydroxide
Examples:
(1)2Na+2H²0→2NaOH+H²+heat
(2)Ca+2H²0→Ca(OH)²+H²
(3)Mg+2H²O→Mg(OH)²+H²
(4)2Al+3H²O→Al²0³+3H²
(5)3Fe+4H²O→Fe³O⁴+4H²
(C) Reaction of metal with Acids(dilute)
Metal +Dilute acid →salt+H²
Cu, Ag, Hg, do not react with dilute acids.
Examples:
(1)Fe+2HCl→FeCl²+H²
(2)Mg+2HCl→MgCl²+H²
(3)Zn+2HCl→ZnCl²+H²
(4)2Al+6HCl→2AlCl³+3H²
(d)Reaction of Metals with solutions of other metal salts:
Metal A+salt solution B→salt solution A+Metal B
•Reaction metals can displace less reactives metals from their compounds in solution form.
Fe+CuSo⁴→FeSo⁴+Cu
Reactivity series
The reactivity series is a list of metals arranged in the order of their decreasing activities.
•Reactivity of elements is the tendency to attain a completely filled valence shell.
•Atoms of the metals lose electrons from their valence shell to from action.
Atom of the non-metals gain electrons
in the valence shell to from anion.
E.g Formation of NaCl
Ionic Compounds
The Compound formed by the transfer of electrons from a metal to a non- metal are called ionic Compounds or electrovalent Compound.
Properties of Ionic Compounds
(I)Physical nature:They are solid and hard , generally brittle.
(II)Melting and Boiling Point:They have high melting and boiling point.
(III)Solubility:Generally soluble in water and insoluble in solvents such as kerosene, petrol etc.
(IV)Conduction of electricity:Ionic Compounds Conduct electricity in molten and solution from but not in solid state.
Occurrence of Metals
Minerals:The elements or Compounds which occur naturally in the earth's crust are called minerals.
Ores:Minerals that Contain very high percentage of particular metal and the metal can be profitably extracted from it such minerals are called ores.
(a)Gangue:Ores are usually Contaminated with large amount of impurities such as soil, sand etc.Called gangue.
(b)Roasting :The sulphide ores are converted into oxides by heating strongly in the presence of excess air. This process is called roasting.
(C)Calcination:The carbonate ores are changed into oxides by heating strongly in limited air .This process is called calcination.
ZnCo³ heat Zno+Co
——›
(d)Reduction:Metal oxides are reduced to corresponding metals by using reducing agent like carbon.
Zno+C→Zn+Co
Refining of Metals
The most widely used method for refining impure metal is electrolytic refining.
•Cathode:strip of pure copper
•Electrolyte:Solution of acidified copper sulphate
(a) On passing the current through electrolyte, the impure metal from anode dissolved into the electrolyte.
(b)An equivalent amount of pure metal from the electrolyte is deposited at the cathode.
(C) The insoluble impurities settle down at the bottom of the anode and is called anode mud.
Corrosion
The surface of some metals such as iron is corroded when they are exposed to moist air for a long period of time.This is called corrosion.
(i) Silver becomes black when exposed to air as it reacts with air to form a coating of silver sulphide.
(ii) Copper reacts with moist carbon dioxide in the air and gains a green coat of copper carbonate.
(iii) Iron when exposed to moist air acquires a coating of a brown flaky substance called rust.
Prevention of Corrosion
The rusting of iron can be prevented by painting, oiling, greasing, galvanizing, chrome plating ,anodizing or making alloys.
Galvanization:It is a method of protecting steel and iron from rusting by coating them with a thin layer of Zinc.
Alloy :An allow is a homogeneous mixture of two or more metals or a non-metal.
Iron:Mixed with small amount of carbon becomes hard and strong.
Steel:Iron+Nickel and chromium
Brass:Copper+Zinc
Bronze:copper+Tin(Sn)
Solder:lead+tin
Amalgam:If one of the metal is Mercury(hg).
Page 53: In-Text Questions
Question 1: Give an example of a metal which:
(a) is a liquid at room temperature.
(b) can be easily cut with a knife.
(c) is the best conductor of heat.
(d) is a poor conductor of heat.
Answer:
(a) Mercury (Hg)
(b) Sodium (Na)
(c) Silver (Ag)
(d) Lead (Pb)
Question 2: Explain the meanings of malleable and ductile.
Answer:
- Malleable: A property of metals where they can be beaten into thin sheets. For example, gold and silver are highly malleable.
- Ductile: A property of metals where they can be drawn into thin wires. For example, copper and aluminium are ductile metals.
Page 55: In-Text Questions
Question 1: Why is sodium kept immersed in kerosene oil?
Answer:
Sodium is a highly reactive metal, and it reacts vigorously with air and water, potentially causing fire. To prevent this, sodium is kept immersed in kerosene oil, which acts as a barrier, preventing any contact with air or moisture.
Question 2: Write equations for the reactions of:
(a) iron with steam
(b) calcium and potassium with water
Answer:
(a) Iron with steam:
3Fe+4H2O(g)→Fe3O4+4H2(b) Calcium with water:
Ca+2H2O→Ca(OH)2+H2Potassium with water:
2K+2H2O→2KOH+H2
Question 3: Samples of four metals A, B, C, and D were taken and added to the following solutions one by one. The results obtained are as follows:
| Metal | Iron(II) sulphate | Copper(II) sulphate | Zinc sulphate | Silver nitrate |
|---|
| A | No reaction | Displacement | No reaction | Displacement |
| B | Displacement | No reaction | No reaction | Displacement |
| C | No reaction | No reaction | No reaction | No reaction |
| D | No reaction | No reaction | No reaction | No reaction |
Use the table above to answer the following questions:
(a) Which is the most reactive metal?
(b) What would you observe if B is added to a solution of copper(II) sulphate?
(c) Arrange the metals A, B, C, and D in the order of decreasing reactivity.
Answer:
(a) Metal B is the most reactive because it displaces iron from iron(II) sulphate and silver from silver nitrate.
(b) If B is added to a solution of copper(II) sulphate, there would be no reaction because B cannot displace copper.
(c) Order of decreasing reactivity: B > A > C ≈ D.
Page 57: In-Text Questions
Question 1: What are amphoteric oxides? Give two examples of amphoteric oxides.
Answer:
Amphoteric oxides are oxides that react with both acids and bases to form salt and water. Examples of amphoteric oxides are aluminium oxide (Al₂O₃) and zinc oxide (ZnO).
Page 58: In-Text Questions
Question 1: Name two metals which will displace hydrogen from dilute acids and two metals which will not.
Answer:
- Metals that will displace hydrogen: Zinc (Zn) and Iron (Fe).
- Metals that will not displace hydrogen: Copper (Cu) and Silver (Ag).
Page 63: Exercises
Question 1: Which of the following pairs will give a displacement reaction?
(a) NaCl solution and copper metal
(b) MgCl₂ solution and aluminium metal
(c) FeSO₄ solution and silver metal
(d) AgNO₃ solution and copper metal
Answer:
(d) AgNO₃ solution and copper metal.
Copper will displace silver from silver nitrate solution, forming copper nitrate and silver metal.
Question 2: Which of the following methods is suitable for preventing an iron frying pan from rusting?
(a) Applying grease
(b) Applying paint
(c) Applying a coating of zinc
(d) All of the above
Answer:
(c) Applying a coating of zinc (Galvanisation) is the most effective method as it protects the iron from rusting more reliably.
Question 3: An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. The element is likely to be:
(a) calcium
(b) carbon
(c) silicon
(d) iron
Answer:
(a) Calcium.
Calcium reacts with oxygen to form calcium oxide (CaO), which has a high melting point and is soluble in water, forming calcium hydroxide.







.jpg)
.jpg)
0 Comments
Use the respectfully words in comment box.